|
Having trouble reading this email?
Download the PDF.

Thematic focus: Ecosystem management
Water hyacinth—can its aggressive invasion be controlled?

The spread of invasive alien species is neither easy to manage nor easy to reverse, threatening not only biodiversity but also economic
development and human wellbeing (UNEP, 2012). Native to the Amazon Basin in South America water hyacinth has emerged as a major weed
in more than 50 countries in the tropical and subtropical regions of the world with profuse and permanent impacts (Patel, 2012,
Téllez et al., 2008, Shanab et al., 2010, Villamagna and Murphy, 2010). Worryingly, climate change may allow the spread of water
hyacinth to higher latitudes (Patel, 2012). Intensified monitoring, mitigation and management measures are needed to keep
water hyacinth at unproblematic levels.
Why is this issue important?
The beautiful, large purple and violet flowers of the South American water hyacinth (Eichhornia crassipes) make it a very
popular ornamental plant for ponds. However water hyacinth has also been labelled as the world’s worst water weed and
has garnered increasing international attention as an invasive species (Zhang et al., 2010). Efficient in utilizing
aquatic nutrients and solar energy for profuse biomass production, water hyacinth can cause extensive environmental,
social and economic problems. It is found in lakes, estuaries, wetlands, marshes, ponds, dambos, slow flowing rivers,
streams, and waterways in the lower latitudes where growth is stimulated by the inflow of nutrient rich water from urban
and agricultural runoff, deforestation, products of industrial waste and insufficient wastewater treatment (Villamagna
and Murphy, 2010, Ndimele et al., 2011). According to recent climate change models, its distribution may expand into higher
latitudes as temperatures rise, posing problems to formerly hyacinth free areas (Rahel and Olden, 2008).
What are the findings?
Invasive alien species are a major global challenge requiring urgent action (Xu et al., 2012). They are considered one of the
key pressures on world’s biodiversity: altering ecosystem services and processes, reducing native species abundance and richness,
and decreasing genetic diversity of ecosystems (Rands et al., 2010, Vila et al., 2011, Hejda et al. 2009). They cause substantial
economic losses estimated by one study to total US$120 billion annually in the USA (Pimentel et al., 2005, Kettunen et al., 2009).
In South Africa, estimated economic costs due to invasive alien species are currently above US$ 700 million (R6.5 billion) per
annum or 0.3% of South Africa’s GDP, and could rise to over 5% of GDP if invasive plants are allowed to reach their full potential
(Wilgen and Lange, 2011).
Water hyacinth has been identified by the International Union for Conservation of Nature (IUCN) as one of the 100 most
aggressive invasive species (Téllez et al., 2008) and recognized as one of the top 10 worst weeds in the world (Shanab et
al., 2010, Gichuki et al., 2012, Patel, 2012). It is characterised by rapid growth rates, extensive dispersal capabilities,
large and rapid reproductive output and broad environmental tolerance (Zhang et al., 2010). In Africa, for example,
where water hyacinth is listed by law as a noxious weed in several countries, it is the most widespread and damaging
aquatic plant species. The economic impacts of the weed in seven African countries have been estimated at between
US$20-50 million every year. Across Africa costs may be as much as US$100 million annually (UNEP, 2006).
The success of this invasive alien species is largely due to its reproductive output. Water hyacinth can flower throughout
the year and releases more than 3 000 seeds per year (Gopal, 1987, EEA, 2012). The seeds are long-lived, up to 20 years
(Gopal, 1987). While seeds may not be viable at all sites, water hyacinth commonly colonises new areas through vegetative
reproduction and propagation of horizontally growing stolons. In the early stages of infestation, the weed takes foothold
on the shoreline in the areas where native aquatic plants thrive (Gichuki et al., 2012). However, it is not restricted to
shallow water, unlike many submersed and emergent macrophytes, because its roots are free-floating near the surface
(Villamagna and Murphy, 2010).

Water hyacinth is found across the tropical and subtropical regions (Figure 1). Originally from the Amazon Basin,
its entry into Africa, Asia, Australia, and North America was facilitated by human activities (Dagno et al., 2012).
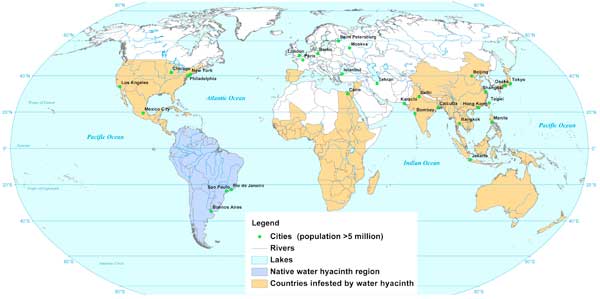
Figure 1. Global distribution of water hyacinth (Map redrawn by UNEP/DEWA from Téllez et al., 2008).
Full Size Image
Africa has particularly been affected by the introduction and spread of water hyacinth, facilitated in part due to a lack of
naturally occurring enemies. In a review of water hyacinth infestation in eastern, southern and central Africa, Mujingni (2012)
reports that the weed was first recorded in Zimbabwe in 1937. It colonized important water bodies, such as the Incomati River in
Mozambique in 1946, the Zambezi River and some important rivers in Ethiopia in 1956. Rivers in Rwanda and Burundi were colonised
in the late 1950s while the rivers Sigi and Pangani in Tanzania were infested in 1955 and 1959. The plant colonised Kafue river
in Zambia in the 1960s, the Shire River in Malawi in 1968 and Lake Naivasha in Kenya in 1986 (Mironga et al., 2012). The plant
was recorded from Lakes Kyoga in Uganda in 1988-89, Victoria in 1989–1990, Malawi/Nyasa in 1996 and Tanganyika in 1997. Lake
Victoria in Africa is the second largest freshwater lake in the world and currently supports approximately 30 million people.
Infestation of water hyacinth in the lake has been a serious nuisance, generating public outcry (World Agro Forestry Centre,
2006, Kateregga and Sterner, 2007, Gichuki et al., 2012). At its peak, it was estimated that the weed was growing at 3 hectares (12 acres)
per day on the lake (Ayodo and Jagero, 2012). The plant also spread fast throughout Uganda’s lakes and rivers in just 10 years.
Water hyacinth has also spread to West Africa. It was first reported in Cameroon between 1997 and 2000 and since then the
country’s wetlands have become "home" for the weed (Forpah, 2009). In Nigeria almost all river bodies have been dominated by
water hyacinth (Borokini and Babalola, 2012). The water hyacinth problem is especially severe on the river Niger in Mali where
human activities and livelihoods are closely linked to the water systems (Dagno et al., 2012). It occurs throughout the Nile
Delta in Egypt and is believed to be spreading southwards, due to the construction of the Aswan Dam which has slowed down the
river flow, enabling the weed to invade (Dagno et al., 2007). Infestation of water hyacinth in Ethiopia has also been manifested
on a large scale in many water bodies of the Gambella area, Lake Ellen in the Rift Valley and Lake Tana (Fessehaie, 2012).
In Europe, water hyacinth is established locally in the Azores (Portugal) and in Corsica (France), and casual records are
known from Belgium, the Czech Republic, Hungary, the Netherlands and Romania (EEA, 2012) In particular, it is a threat in
Spain and Portugal (DellaGreca et al., 2009).
In Asia, water hyacinth is widespread on freshwater wetlands of the Mekong Delta, especially in standing water (MWBP/RSCP, 2006).
It has been detected in the Sundarbans mangrove forest of Bangladesh (Biswas et al., 2007) and has caused heavy siltation in the
wetlands of the Kaziranga National Park, India. Deepor Beel, a freshwater lake formed by the Brahmaputra River is heavily infested
with this weed (Patel, 2012). The lake is considered one of the large and important riverine wetlands in the Brahmaputra valley of
lower Assam, India. As in many other countries, water hyacinth has caused many economic, social and environmental problems in
southern China (Choo et al., 2006).
In Mexico, more than 40 000 hectares of reservoirs, lakes, canals and drains are infested with water hyacinth
(Jimenez and Balandra, 2007). In California, USA, this weed has caused severe ecological impacts in the Sacramento- San
Joaquin River Delta (Khanna et al., 2011).
Threats posed by water hyacinth
i. Destruction of biodiversity
Today, biological alien invasions are a major driver of biodiversity loss worldwide, (Pyšek and Richardson, 2010,
Vila et al., 2011). Water hyacinth is challenging the ecological stability of freshwater water bodies (Khanna et al.,
2011, Gichuki et al., 2012), out-competing all other species growing in the vicinity, posing a threat to aquatic
biodiversity (Patel, 2012). Besides suppressing the growth of native plants and negatively affecting microbes, water
hyacinth prevents the growth and abundance of phytoplankton under large mats, ultimately affecting fisheries
(Gichuki et al., 2012, Villamagna and Murphy, 2010).
ii. Oxygen depletion and reduced water quality
Large water hyacinth mats prevent the transfer of oxygen from the air to the water surface, or decrease oxygen
production by other plants and algae (Villamagna and Murphy, 2010). When the plant dies and sinks to the bottom the
decomposing biomass depletes oxygen content in the water body (EEA, 2012). Dissolved oxygen levels can reach dangerously
low concentrations for fish that are sensitive to such changes. Furthermore, low dissolved oxygen conditions catalyse
the release of phosphorus from the sediment which in turn accelerates eutrophication and can lead to a subsequent
increase in water hyacinth or algal blooms (Bicudo et al., 2007). Death and decay of water hyacinth vegetation in large
masses deteriorates water quality and the quantity of potable water, and increases treatment costs for drinking water
(Patel, 2012, Mironga et al., 2011, Ndimele et al., 2011).
iii. Breeding ground for pests and vectors
Floating mats of water hyacinth support organisms that are detrimental to human health. The ability of its mass of fibrous,
free-floating roots and semi-submerged leaves and stems to decrease water currents increases breeding habitat for the malaria
causing anopheles mosquito as evidenced in Lake Victoria (Minakawa et al., 2008). Mansonioides mosquitoes, the vectors of human
lymphatic filariasis causing nematode Brugia, breed on this weed (Chandra et al., 2006, Varshney et al., 2008). Snails serving as
vector for the parasite of Schistosomiasis (Bilharzia) reside in the tangled weed mat (Borokini and Babalola, 2012). Water hyacinth
has also been implicated in harbouring the causative agent for cholera. For example, from 1994 to 2008, Nyanza Province in Kenya, which
borders Lake Victoria accounted for a larger proportion of cholera cases than expected given its population size (38.7% of cholera
cases versus 15.3% of national population). Yearly water hyacinth coverage on the Kenyan section of the lake was positively
associated with the number of cholera cases reported in the Province (Feikin et al., 2010). At the local level increased
incidences of crocodile attacks have been attributed to the heavy infestation of the weed which provides cover to the
reptiles and poisonous snakes (Patel, 2012, Ndimele et al., 2011).
iv. Blockage of waterways hampering agriculture, fisheries, recreation and hydropower
Water hyacinth often clogs waterways due to its rapid reproduction and propagation rate. The dense mats disrupt socioeconomic
and subsistence activities (ship and boat navigation, restricted access to water for recreation, fisheries, and tourism) if
waterways are blocked or water pipes clogged (Ndimele et al., 2011, Patel, 2012). The floating mats may limit access to breeding,
nursery and feeding grounds for some economically important fish species (Villamagna and Murphy, 2010). In Lake Victoria, fish
catch rates on the Kenyan section decreased by 45% because water hyacinth mats blocked access to fishing grounds, delayed access
to markets and increased costs (effort and materials) of fishing (Kateregga and Sterner, 2009). In the Wouri River Basin in Cameroon
the livelihood of close to 900,000 inhabitants has been distorted; the entire Abo and Moundja Moussadi creeks have been rendered
impassable by the weed leading to a complete halt in all the socioeconomic activities with consequent rural exodus (Mujingni, 2012).
The weed has made navigation and fishing an almost impossible task in Nigeria (Ndimele et al., 2011).
While navigation in the Brahmaputra River in India has been affected by the weed, it has also blocked irrigation channels and
obstructed the flow of water to crop fields (Patel, 2012). For example, in West Bengal, it causes an annual loss of paddy (Patel,
2012) by directly suppressing the crop, inhibiting rice germination and interfering with harvesting (EEA, 2012). The dense growth
entangles with boat propellers, hampering fishing (Patel, 2012). Water hyacinth slows water flow by 40 to 95% in irrigation
channels (Jones, 2009), which may cause severe flooding. The communities of Bwene and Bonjo in the Wouri River Basin in Cameroon
regularly suffer from floods during the rainy season due to blockage of waterways around the villages by the weed (Mujingni, 2012).
It is estimated that the flow of water in the Nile could be reduced by up to one tenth due to increased losses from
evapotranspiration by water hyacinth in Lake Victoria (Ndimele et al., 2011). Water loss by the same process and blocking of
turbines on Kafue Gorge in Zambia translates into lost water for power generation and eventually into lost revenue of about US$15
million every year for the power company (ZEO, 2008). Many large hydropower schemes are also suffering the effects of water hyacinth
(Shanab et al., 2010). For example, cleaning intake screens at the Owen Falls hydroelectric power plant at Jinja in Uganda were
calculated to be US$1 million per annum (Mailu, 2001).
Control measures
Water hyacinth control is absolutely essential (Villamagna and Murphy, 2010). Control methods that are often used include
mechanical, chemical and biological control. However, existing methods have often been insufficient to contain the aggressive
propagation of the weed and viability of its seeds despite substantial monetary investments over the years (Gichuki et al., 2012),
mainly due to lack of continued policy and management support by governments. The weed infestation on Lake Chivero which supplies
water to Harare, Zimbabwe, was controlled and declined from 42% in 1976 to 22% in 2000. Re-invasion began to emerge in 2005 and
included massive amounts of another invasive plant, spaghetti weed (Hydrocotyle ranunculoide (UNEP, 2008). The Oct 2012
image shows the extent of re-invasion (Figure 2).
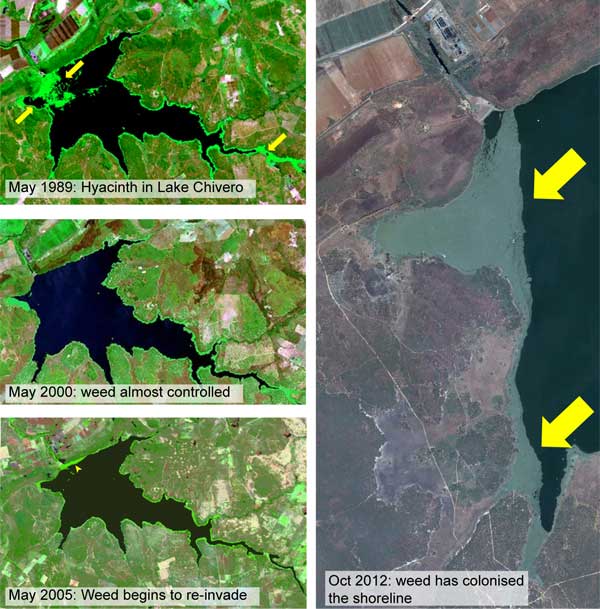
Figure 2: Satellite images showing progressive invasion, control and re-invasion of water hyacinth on Lake Chivero, Zimbabwe
(Image source: Google Earth and Landsat).
Full Size Image
i. Manual and mechanical control
Physical methods for control of water hyacinth involve drainage of the water body, manual removal of the weeds or pulling
through nets (Patel, 2012). Employing machines like weed harvesters, crusher boats, and destruction boats prove expensive,
approximately US$600-1,200 per hectare (Malik, 2007, Villamagna and Murphy, 2010) as well as unpractical for areas larger than a
hectare given the rapid rate of increase of the weed. There may also be additional fees for disposal of plant material. The costs
of water hyacinth management in China were estimated to amount around EUR 1 billion annually (EEA, 2012). In Europe, management
costs to remove 200,000 tonnes of the plant along 75 km in the Guadiana river basin on the Portuguese-Spanish border amounted to
EUR 14,680,000 between 2005 and 2008 (EEA, 2012). Dagno et al. (2007) reported that mechanical management of the weed in Mali cost
around US$ 80,000–100,000 per year. Maintaining a clear passage for ships to dock at Port Bell in Uganda is estimated to cost
US$ 3-5 million per year (Mailu, 2001). Yet, while mechanical removal has been effective to a considerable extent, the infestations
soon return because shredded bunches of the weed are carried by waves to other unaffected areas where they establish and start
proliferating (Shanab et al., 2010).

ii. Chemical control
A generally cheaper method has been used worldwide to reduce water hyacinth populations through the use of chemical herbicides
(such as Paraquat, Diquat, Glyphosate, Amitrole, 2, 4-D acid) (Villamagna and Murphy, 2010). However, their use directly interferes with the
biocontrol agents currently deployed against this weed. Long term use may degrade water quality and put aquatic life at risk (Malik, 2007)
with significant socio-economic impacts if beneficial or designated uses of the water body such as drinking and preparing food are
affected (Dagno et al., 2012). Considering that hundreds of thousands of hectares have been invaded by the weed, it is unlikely that
it will be controlled by chemical means alone (Borokini and Babalola, 2012).
iii. Biological control
In recent years, focus has shifted to natural enemies of water hyacinth including plant pathogens (Dagno et al., 2012, Villamagna
and Murphy, 2010). The aim of any biological control is not to eradicate the weed, but to reduce its abundance to a level where
it is no longer problematic. While there exists several native enemies of water hyacinth, two South American weevil beetles
(Neochetina eichhorniae and Neochetina bruchi) and two water hyacinth moth species (Niphograpta albiguttalis and Xubida infusella) have
had effective long-term control of water hyacinth in many countries, notably at Lake Chivero (Zimbabwe), Lake Victoria (Kenya), Louisiana
(USA), Mexico, Papua New Guinea and Benin (Williams et al., 2007, Venter et al., 2012, Gichuki et al., 2012, Dagno et al., 2012). Researchers
have identified another tiny insect, Megamelus scutellaris, from South America which is highly host-specific to water hyacinth and
does not pose a threat to native or economically important species (Coetzee et al., 2009).
The weevils reduce water hyacinth vigour by decreasing plant size, vegetative reproduction, and flower and seed production.
They also facilitate the transfer and ingress of deleterious microorganisms associated with the weevils (both fungi and bacteria)
into the plant tissues (Venter et al., 2012).
Control of water hyacinth using fungal pathogens has greatly stimulated interest in the management of the weed. Several
fungal species among theme Cercospora rodmanii, Alternaria alternata and A. eichhorniae are recognized as potential
mycoherbicide agents although no commercial mycoherbicide is available for water hyacinth (Dagno et al., 2012).
|
Box 1. An example of the benefits of biological control
Between 1991 and 1993, a biological control program of water hyacinth was undertaken in Southern Benin. It consisted of
the release of three natural enemies, two weevil species (these are the two Neochetina spp.) and one moth that feed
exclusively on water hyacinth. In 1999, a survey of 365 men and women from 192 households in 24 villages in the target area
revealed that water hyacinth, although not eliminated, was perceived by the villagers as having been reduced from a serious pest
to one of minor or moderate importance. At the peak of the infestation water hyacinth had reduced the yearly income of this
population of about 200 000 by approximately US$84 million. Lost revenues for men were mostly in fishing, while women experienced
lost revenues in trade, primarily food crops and fish. The reduction of water hyacinth cover through biological control was
credited with an increase in income of US$30.5 million per year. The total cost of the control program is estimated at a
present value of US$2.09 million. The benefits therefore appear to outweigh the costs by a ratio of 124:1 (De Groote et al., 2003).
|

|
iv. Reduction by utilisation
Research into the utilisation and related technologies for the control of water hyacinth have been tested over the last few
decades (Ndimele et al., 2011). It is being speculated that the biomass can be used in waste water treatment, heavy metal and
dye remediation, as substrate for bioethanol and biogas production, electricity generation, industrial uses, medicines, animal
feed, agriculture and sustainable development (Patel, 2012). However, seldom does utilisation provide a sustained solution to the
spread and impact of water hyacinth, and in fact could provide a perverse incentive to maintain the invasive plant to the detriment
of the environment and production systems at high economic and social costs. There is not one example from anywhere in the
world where utilisation alone has contributed to the management of any invasive plant (EEA, 2012).
Waste water treatment and clean-up of polluted environment
Water hyacinth has the potential to clean up various contaminated waters (Mahamadi and Nharingo, 2010, Rahman and Hasegawa, 2011,
Smolyakov, 2012). It can be used to treat wastewater from dairies, tanneries, sugar factories, pulp and paper industries,
palm oil mills, distilleries etc. (Jafari, 2010). The plant can absorb into its tissues large quantities of heavy metals
from the water column and grows very well in water polluted with organic contaminants and high concentrations of plant
nutrients (Chunkao et al., 2012, Ndimele, 2012). In the Ologe Lagoon, Nigeria, water hyacinth that was not deliberately
introduced into the lagoon to absorb heavy metals did so, even when the concentration of the heavy metals in the water
column was very small (Ndimele and Jimoh, 2011). In California, water hyacinth leaf tissue was found to have the same
mercury concentration as the sediment beneath, suggesting that plant harvesting could help mediate mercury contamination
(Greenfield et al., 2007). While water hyacinth’s capacity to absorb nutrients makes it a potential biological
alternative for treatment of agro-industrial wastewater, one of the major challenges is how to properly
dispose the vast amount of the plant materials which may have to be considered as toxic waste (Zhang, 2012).
As alternative fuel and energy source
Water hyacinth fulfills all the criteria deemed necessary for bioenergy production – it is perennial, abundantly
available, non-crop plant, biodegradable and has high cellulose content; however its strong disadvantage is that
it has over 90% water content which complicates harvesting and processing. The biomass can be subjected to biogas
production to generate energy for household uses in rural areas (Chuang et al., 2011). Experiments in China show
that mixing biomass of water hyacinth with pig manure leads to a much higher biogas production than by using pig
manure alone (Lu et al., 2010). It can also be used for producing ethanol, but technical and logistical challenges
need to be overcome before the commercial scale ethanol production becomes a reality because of the high
tissue water content (Ndimele et al., 2011).
Semi-industrial uses and household articles
As a readily available resource, water hyacinth has been used in several small cottage industries in the Philippines,
Indonesia and India for paper, rope, basket, mats, shoes, sandals, bags, wallets, vases, etc (Ndimele et al., 2011,
Patel, 2012). Yet these are rarely successful to reduce infestations and the market for these products is far too
small to have any impact on water hyacinth populations. In addition, income generation may facilitate its spread
to new, uninvaded, water bodies.
Animal feedstock and agricultural use
When sun-dried, water hyacinth has been found to be rich in protein, vitamins and minerals and serves as a high
quality feedstock for some non-ruminant animals, poultry and fishery in Indonesia, China, the Philippines and Thailand
(Lu et al., 2010, Saha and Ray, 2011). But it is not recommended for use if primarily used for removal of heavy metals
and toxic substances from wastewater (Chunkao et al., 2012). Decomposed water hyacinth can also be used as green manure
or as compost that improves poor quality soils (Ndimele et al., 2011). However, its high alkalinity (pH>9) and potentially
toxic heavy metals contents would restrict its use to flowering-plants, with no allowable application to horticulture
for edible vegetables (Chunkao et al., 2012, Zhang, 2012).
What are the implications for policy?
Water hyacinth infestation is a symptom of broader watershed management and pollution problems. It calls for a concise
national and transboundary water hyacinth policy designating the plant as noxious weed to aquatic systems. In October
2010, world leaders adopted the Strategic Plan for Biodiversity (2011–2020) targeting the need for identification of
invasive alien species and pathways, the need to control and eradicate priority species, and to manage
pathways in order to prevent further invasions (CBD, 2010).
Given the complexity of control options and the potential for climate change to assist the spread of water hyacinth,
it is critical to develop comprehensive management strategies and action plans. A multidisciplinary approach should be
designed, which ensures that the highest political and administrative levels recognize the potential seriousness of the
weed. Plans should also state clearly the role of each government department, stakeholders, municipal councils and
local community involved in the fight against water hyacinth.
Awareness needs to be raised amongst local communities and all stakeholders on the inherent dangers of water hyacinth
infestation to mobilize riparian communities towards control measures. One practical approach is to involve
communities in manual and biological control activities, for example, in rearing weevils. There are excellent examples
of community involvement in the rearing and distribution of the weevils to control the hyacinth around Lake Victoria.
Methods for water hyacinth control should include reduction of nutrient load in the water bodies through treatment
of waters flowing from sewage works, urban wastes and factories. Changing land use practices in the riparian communities
through watershed management will help reduce agricultural runoff as a mechanism for controlling the proliferation of
water hyacinth. This is considered by many as one of the most sustainable long-term management actions.
In order for policy makers to make informed decisions, much more economic information is required on the costs
and benefits of environmental programs. For example, it is frequently stated that there are insufficient resources
to control hyacinth. However, if the costs of improved water treatment are compared with the costs of decreased
fish catches and the costs of increased water-borne diseases, it is likely that resources needed for
hyacinth control are modest in comparison to potential losses from its proliferation (see Box 1).
While researchers continue to investigate the perceived potential uses of water hyacinth, the current
negative impacts of the weed far outweigh its benefits. The use of water hyacinth as raw material in
cottage industry should not encourage propagation of the weed, but rather help control its growth.
Acknowledgement
Written by: Mwangi Theuria.
Production and Outreach Team: Arshia Chanderb, Erick Litswaa, Kim Gieseb, Lindsey Harrimanb,
Michelle Anthonyb, Reza Hussainb, Mwangi Theuria and Zinta Zommersa
Special thanks to Zinta Zommersa, Anna Stabrawaa, Frank Turyatungaa, Neeyati Patela, Max Zierenc,
Maxwell Gomerad and Arne Witte for their valuable inputs and review.
(a UNEP/DEWA/Nairobi, b UNEP/GRID-Sioux Falls, c UNEP-ROAP, d UNEP-WCMC, e CAB International-Nairobi)
References
Ayodo, T. and Jagero, N., 2012. The economic, educational and social responsibilities of elders development groups in lake Victoria region. Academic Research International, 2 (3):610-620.
Bicudo, D., Fonseca, B., Bini, L., Crossetti, L., Bicudo, C. and Araujo-Jesus, T., 2007. Undesirable side-effects of water hyacinth control in a shallow tropical reservoir. Freshwater Biology, 52, 1120–1133.
Biswas, S., Choudhury, J., Nishat, A., Rahman, M., 2007. Do invasive plants threaten the Sundarbans mangrove forest of Bangladesh? Forest Ecol Manag 245:1–9.
Borokoni, T. and Babalola, F., 2012. Management of invasive plant species in Nigeria through economic exploitation: lessons from other countries. Management of Biological Invasions 3 (1): 45–55 doi: http://dx.doi.org/10.3391/mbi.2012.3.1.05.
CBD, 2010. Strategic Plan for Biodiversity 2011–2020. Secretariat of the Convention on Biological Diversity, Montreal.
http://www.cbd.int/decision/cop/?id=12268 (accessed 23 April 2013).
Chandra, G., Ghosh, A., Biswas, D. and Chatterjee, S., 2006. Host plant preference of Mansonia mosquitoes. J Aquatic Plant Manage 44:142–144.
Choo, T., Lee, C., Low, K. and Hishamuddin, O., 2006. Accumulation of chromium (VI) from aqueous solutions using water lilies (Nymphaea spontanea). Chemosphere 62:961–996.
Chuang, Y-S., Lay, C-H., Sen, B., Che,n C-C., Gopalakrishnan,. K., Wu, J-H., Lin, C-S. and Lin, C-Y., 2011. Biohydrogen and biomethane from water hyacinth (Eichhornia crassipes)
fermentation: effects of substrate concentration and incubation temperature. Int J Hydr Energy 36:14195–14203.
Chunkao, K., Nimpee, C, and Duangmal, 2012. The King's initiatives using water hyacinth to remove heavy metals and plant nutrients from wastewater through Bueng Makkasan in Bangkok, Thailand. Ecological Engineering 39: 40–52.
Coetzee, J., Hill, M., Julien, M., Center, T. and Cordo, H., 2009. Eichhornia crassipes (Mart.) Solms–Laub. (Pontederiaceae).
In: Biological Control of Tropical Weeds using Arthropods, (ed). R. Muniappan, G. V. P. Reddy, and A. Raman. Cambridge University Press, Cambridge. 183-210.
Dagno, K., Lahlali, R., Diourte, M., and Haissam, J., 2012. Fungi occurring on waterhyacinth (Eichhornia crassipes [Martius] Solms-Laubach) in Niger River in Mali and their evaluation as Mycoherbicides. J. Aquat. Plant Manage. 50: 25-32.
Dagno, K., Lahlali, R., Friel, D., Bajji, M. and Jijakli, H., 2007. Review: problems of the water hyacinth, Eichhornia crassipes in the tropical and subtropical areas of the world,
in particular its eradication using biological control method by means of plant pathogens. Biotechnol. Agron. Soc. Environ. 11 (4): 299-311.
De Groote, H., Ajuonua, O, Attignona, S, Djessoub, R, and Neuenschwandera, P (2003). Economic impact of biological control of water hyacinth in Southern Benin. Ecological Economics, 45 (1): 105 – 117.
DellaGreca, M., Previtera, L. and Zarrelli, A., 2009. Structures of new phenylphenalene-related compounds from Eichhornia crassipes (water hyacinth). Tetrahedron 65:8206–8208.
EEA, 2012. The impacts of invasive alien species in Europe. EEA Technical report No 16/2012. Luxembourg: Publications Office of the European Union,
2012. http://www.eea.europa.eu/publications/impacts-of-invasive-alien-species (accessed 12 March 2013).
Feikin, D., Tabu, C. and Gichuki, J., 2010. Does water hyacinth on East African lakes promote cholera outbreaks? American Journal of Tropical Medicine and Hygiene 83: 370–373. doi:10.4269/ajtmh.2010.09-0645.
Fessehaie, R., 2012. Status of water hyacinth (Eichhornia crassipes) in Ethiopia: Challenges and response. In: Berihun Tefera, Workiye Worie and Melaku Wale(eds.).
Proceedings of the Second National Workshop on Challenges and Opportunities of Water Resources Management in Tana Basin, Upper Blue Nile Basin, Ethiopia, 26 – 27 March 2012.
Blue Nile Water Institute - Bahir Dar University (BNWI-BDU), Bahir Dar, Ethiopia, 159-166.
Forpah, N., 2009. Cameroon prepares a National Strategy for the control of water hyacinth (exotic species). Workshop proceedings on the elaboration of a national strategy
for the control of water hyacinth in Cameroon, 15th – 18th September 2009, Douala, Cameroon.
http://wtgpartners.org/ (accessed 12 March 2013).
Gichuki, J., Omondi, R., Boera, P., Tom Okorut, T., SaidMatano, A., Jembe, T. and Ofulla, A., 2012. Water Hyacinth Eichhornia crassipes (Mart.) Solms-Laubach Dynamics
and Succession in the Nyanza Gulf of Lake Victoria (East Africa): Implications forWater Quality and Biodiversity Conservation. The ScientificWorld Journal Volume 2012, Article ID 106429, 10 pages doi:10.1100/2012/106429.
Gopal, B., 1987. Aquatic Plant Studies 1. Water hyacinth. Elsevier, Amsterdam.
Greenfield, B.K., Siemering, G.S., Andrews, J.C., Rajan, M., Andrews, S.P., Spencer, D.F., 2007. Mechanical
Shredding of Water Hyacinth (Eichhornia crassipes). Estuaries and Coasts. 30(4), 627 - 640.
Hejda, M., Pyšek, P. and Jarošík, V., 2009. Impact of invasive plants on the species richness, diversity and composition of invaded communities. Journal of Ecology 97: 393–403.
Jafari, N., 2010. Ecological and socio-economic utilization of water hyacinth (Eichhornia crassipes Mart Solms). J Appl Sci Environ Manag 14:43–49.
Jimeonez, M. and Balandra, M., 2007. Integrated control of Eichhornia crassipes by using insects and plant pathogens in Mexico. Crop Prot 26:1234–1238.
Jones, R., 2009. The impact on biodiversity, and integrated control, of water hyacinth, Eichhornia crassipes (Martius) Solms-Laubach (Pontederiaceae) on the Lake
Nsezi –Nseleni River System. Mc Thesis. Department of Zoology and Entomology-Rhodes University. South Africa. 115p.
Kateregga, E., and Sterner, T., 2007. Indicators for an invasive species: water hyacinths in Lake Victoria. Ecol Indic 7:362–370.
Kateregga, E. and Sterner, T., 2009. Lake Victoria fish stocks and the effects of water hyacinth. The Journal of Environment & Development, 18, 62–78.
Kettunen, M., Genovesi, P., Gollasch, S., Pagad, S., Starfinger, U., ten Brink, P. and Shine, C., 2009. Technical support to EU strategy on invasive species (IAS):
assessment of the impacts of IAS in Europe and the EU (final module report for the European Commission). Institute for European Environmental Policy, Brussels.
Khanna, S., Santos, M., Ustin, S., Haverkamp, P., 2011. An integrated approach to a biophysiologically based classification of floating aquatic macrophytes. Int J Remote Sens 32:067–1094.
Lu, J., Zhu, L., Hu, G. and Wu, J., 2010. Integrating animal manure-based bioenergy production with invasive species control: A case study at Tongren pig farm in China. Biomass Bioenerg 34: 821–827. doi: 10.1016/j.biombioe.2010.01.026.
Mahamadi, C. and Nharingo, T., 2010. Competitive adsorption of Pb2+, Cd2+ and Zn2+ ions onto Eichhornia crassipes in binary and ternary systems. Bioresour Technol 101:859–864.
Mailu, A., 2001. Preliminary assessment of the social, economic and environmental impacts of water hyacinth in the Lake Victoria basin and the status of control.
In: Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes. ACIAR Proceedings No. 102.
Malik, A., 2007. Environmental challenge vis a vis opportunity: the case of water hyacinth. Environ Int 33:122–138.
Minakawa, N., Sonye, G., Dida, G., Futami, K. and Kaneko, S., 2008. Recent reduction in the water level of Lake Victoria has created more habitats for Anopheles funestus. Malaria J 7:119.
Mironga, J., Mathooko, J. and Onywere, S., 2011. The Effect of Water Hyacinth (Eichhornia Crassipes) Infestation on Phytoplankton Productivity in
Lake Naivasha and the Status of Control. Journal of Environmental Science and Engineering 5(10) 1252-1261.
Mironga, J., Mathooko, J. and Onywere, S., 2012. Effect of Water Hyacinth Infestation on the Physicochemical Characteristics of Lake Naivasha. International Journal of Humanities and Social Science 2(7) 103-113.
Mujingni, C., 2012. Quantification of the impacts of Water Hyacinth on riparian communities in Cameroon and assessment of an appropriate method of control: The case of the
River Wouri Basin: The Case of the Wouri River Basin. Msc disseratation. World Maritime University, Malmö, Sweden.
MWBP/RSCP, 2006. Invasive Alien Species in the Lower Mekong Basin: Current State of Play. Mekong Wetland Biodiversity Programme and Regional Species Conservation Programme,
The World Conservation Union (IUCN), Asia, Sri Lanka; 22pp.
Ndimele, P., Kumolu-Johnson, C. and Anetekhai, M. 2011. The invasive aquatic macrophyte, water hyacinth {Eichhornia crassipes (Mart.) Solm-Laubach: Pontedericeae}: problems and prospects. Res J Environ Sci 5:509–520.
Ndimele, P. 2012. The Effects of Water hyacinth (Eichhornia crassipes [Mart.] Solms) Infestation on the Physico-Chemistry, Nutrient and Heavy Metal Content of Badagry Creek and Ologe Lagoon, Lagos, Nigeria.
Journal of Environmental Science and Technology, 5, 128-136. DOI: 10.3923/jest.2012.128.136.
Ndimele, P. and Jimoh, A., 2011. Water Hyacinth (Eichhornia crassipes [Mart.] Solms.) in Phytoremediation of heavy Metal Polluted Water of Ologe lagoon, Lagos, Nigeria.
Research journal of Environmental Sciences, 5(5), 424-433. DOI: 10.3923/rjes.2011.424.433.
Patel, S. , 2012. Threats, management and envisaged utilizations of aquatic weed Eichhornia crassipes: an overview. Rev Environ Sci Biotechnol (2012) 11:249–259. DOI 10.1007/s11157-012-9289-4.
Pimentel, D., Zuniga, R. and Morrison, D., 2005. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecological Economics 52(3), 273–288.
Pyšek, P., and Richardson, D., 2010. Invasive species, environmental change and management, and health. Annual Review of Environment and Resources 35: 25–55. doi: 10.1146/annurevenviron- 033009-095548.
Rahel, F. and Olden, J., 2008. Assessing the effects of climate change on aquatic invasive species. Conservation Biology, 22, 521–533.
Rahman, M. and Hasegawa, H., 2011. Aquatic arsenic: phytoremediation using floating macrophytes. Chemosphere 83:633–646.
Rands, M., Adams, W., Bennun, L., Butchart, S., Clements, A., Coomes, D., Entwistle, A., Hodge, I., Kapos, V., Scharlemann, J., Sutherland, W. and Vira, B., 2010. Biodiversity conservation: Challenges beyond 2010. Science 329: 1298–1303.
Saha, S., Ray, A.K., 2011. Evaluation of Nutritive Value of Water Hyacinth (Eichhornia crassipes) Leaf Meal in
Compound Diets for Rohu, Labeo rohita (Hamilton, 1822) Fingerlings after Fermentation with Two Bacterial Strains
Isolated from Fish Gut. Turkish Journal of Fisheries and Aquatic Sciences. 11, 199 - 207.
Shanab, S,, Shalaby, E., Lightfoot, D. and El-Shemy, H., 2010. Allelopathic effects of water hyacinth (Eichhornia crassipes). PLoS One 5(10):e13200. doi:10.1371/journal.pone.0013200.
Smolyakov, B., 2012. Uptake of Zn, Cu, Pb, and Cd by water hyacinth in the initial stage of water system remediation. Appl Geochem. 27(6), 1214 – 1219. doi:10.1016/j.apgeochem.2012.02.027.
Téllez, T., López, E., Granado, G., Pérez, E., López, R., and Guzmán, J., 2008. The water hyacinth, Eichhornia crassipes: an invasive plant in the Guadiana River Basin (Spain). Aquatic Invasions 3, 42-53.
UNEP, 2006. Africa Environment Outlook 2. Division of Early Warning and Assessment, United Nations Environment Programme, Nairobi.
UNEP, 2008. Africa Atlas of our changing environment Division of Early Warning and Assessment (DEWA). United Nations Environment Programme, Nairobi.
UNEP, 2012. Fifth Global Environment Outlook (GEO5): Environment for the future we want. United Nations Environment Programme, Nairobi.
Varshney, J., kumar, S., Mishra, J., 2008. Current status of aquatic weeds and their management in India. In: Proceedings of Taal2007: the 12th world lake conference, pp 1039–1045.
Venter, N., Hill, M., Hutchinson, S. and Ripley, B., 2012. Weevil borne microbes contribute as much to the reduction of photosynthesis in water hyacinth as does herbivory. Biological Control 64 (2013) 138–142.
Vila, M., Espinar, J., Hejda, M., Hulme, P., Jarošík, V., Maron, J., Pergl, J., Schaffner, U., Sun, Y. and Pyšek, P., 2011. Ecological impacts of invasive
alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecology Letters 14: 702–708.
Villamagna, A. and Murphy, B., 2010. Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): a review. Freshwater Biology (2010) 55, 282–298 doi:10.1111/j.1365-2427.2009.02294.x
Wilgen, B, and Lange, W., 2011. The costs and benefits of biological control of invasive alien plants in South Africa. African Entomology 19(2): 504–514.
Williams, A., Hecky, R., Duthie, H., 2007. Water hyacinth decline across Lake Victoria-Was it caused by climatic perturbation or biological control? A reply. Aquatic Bot 87:94–96.
World Agroforestry Centre, 2006. Improved Land Management in Lake Victoria Basin: ICRAF Occasional paper No. 7. Nairobi; World Agro Forestry Centre.
Xu, H., Qiang, S., Genovesi, P., Ding, H., Wu, J., Meng, L., Han, Z., Miao, J., Hu, B., Guo, J., Sun, H., Huang, C., Lei, J., Le, Z., Zhang, X., He, S., Wu, Y., Zheng, Z.,
Chen, L., Jarošík, V. and Pyšek, P., 2012. An inventory of invasive alien species in China. NeoBiota 15: 1–26. doi: 10.3897/neobiota.15.3575.
ZEO, 2008. Zambia Environment Outlook Report 3. Environmental Council of Zambia, 201pp.
Zhang H (2012). Can Water Hyacinth Clean Highly Polluted Waters? —A Short Paper for Discussion. Journal of Environmental Protection 3: 340-341 doi:10.4236/jep.2012.34043.
Zhang, Y., Zhang, D., Barrett, S., 2010. Genetic uniformity characterises the invasive spread of water hyacinth (Eichhornia crassipes), a clonal aquatic plant. Molecular Ecology 19: 1774-1786.
If you no longer wish to receive this bulletin you can unsubscribe anytime.
Information is regularly scanned, screened, filtered, carefully edited, and published for educational purposes. UNEP does not accept any liability
or responsibility for the accuracy, completeness, or any other quality of information and data published or linked to the site. Please read our
privacy policy and
disclaimer for further information.
|











